SMH Joins International Study of Novel Heart Failure Treatment
Business
SRQ DAILY THURSDAY FAMILY AND RECREATION EDITION
THURSDAY JUL 27, 2023 |
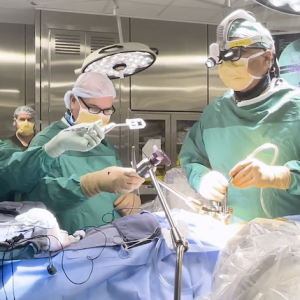
Despite decades of advances in the understanding and treatment of heart failure, standard therapies do not work well for all patients, leaving many with chronic and at times life-threatening symptoms. An investigational cardiac implant, however, is raising the possibility of a new treatment option and hope for heart failure patients whose symptoms cannot be controlled by current therapies. Corvia Medical’s RESPONDER-HF study, now under way at Sarasota Memorial Hospital and about 60 other sites in the United States, Europe, and Australia, gives qualifying heart failure patients randomized access to atrial shunt therapy. The Corvia® Atrial Shunt is a minimally invasive cardiac implant that has been shown to reduce symptoms and improve quality of life. In earlier safety studies, atrial shunting resulted in significant reductions in the rate of heart failure hospitalizations and sustained improvement in patients’ quality of life. Sarasota Memorial’s Research Institute is currently enrolling patients in the RESPONDER-HF confirmatory study. Led by Sarasota cardiologist Hakim Morsli, MD, the local study is enrolling heart failure patients with preserved ejection fraction (HFpEF), the most common type of heart failure, but one for which effective treatments are limited. More than 26 million people worldwide have HF, and the majority have HFpEF, making it the largest unmet clinical need in cardiovascular medicine. The atrial shunt is placed by an interventional cardiologist or electrophysiologist during a one-time minimally invasive procedure. A catheter is used to place the shunt as a passage between the left and right atria. This passage allows blood to flow from the high pressure left atrium to the lower pressure right atrium, relieving pressure in the heart and lungs and thereby reducing shortness of breath, chest pain and other symptoms that put many HFpEF patients at risk. The RESPONDER-HF is a randomized, double-blind, sham-controlled study that will enroll up to 260 patients, age 40 or older, whose heart failure symptoms persist despite directed medical therapy. Patients will be randomized to a treatment track (to receive the investigational device) or a control track (where they will undergo testing and examination but will not receive the device), and monitored for five years. Neither the participants nor researchers know which treatment participants receive until their 24-month follow-up visit. The Corvia Atrial Shunt is the most widely studied atrial shunt for heart failure. It has been implanted in more than 550 patients worldwide and reviewed in academic publications. The RESPONDER-HF trial builds on the extensive data and progressive learnings from the earlier REDUCE LAP-HF II clinical trial, the largest randomized controlled trial of device-based therapy in HFpEF. Results of that study showed that within a large responder population, representing 50% of study patients, treatment with the Corvia Atrial Shunt resulted in a 45% reduction in heart failure events and a 55% greater improvement in quality of life compared to sham control.
« View The Thursday Jul 27, 2023 SRQ Daily Edition
« Back To SRQ Daily Archive